
Antares Pharma Drug Portfolio
Antares Pharma focuses on the development and commercialization of self-administered pharmaceutical products and the technologies to deliver them. We have multiple internal product development programs, as well as partnerships with industry-leading pharmaceutical companies.
XYOSTED®
XYOSTED® is the first and only weekly auto-injector testosterone therapy.
TLANDO™
TLANDO™ is an oral treatment for testosterone replacement therapy.
Sumatriptan
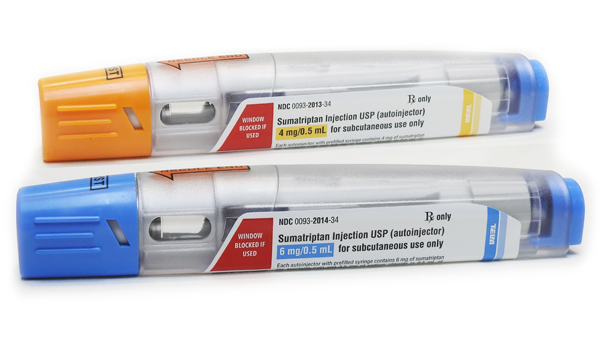
In June 2016 we launched the generic equivalent to Imitrex® (sumatriptan succinate) injection, 4 mg and 6 mg single-dose, prefilled syringe autoinjectors, in the U.S. with our distribution partner Teva Pharmaceuticals. Sumatriptan injection is used to treat acute migraine with or without aura, and acute cluster headaches in adults.
Sumatriptan Injection USP represents Antares first ANDA approval of a complex generic and second product approved using the VIBEX® auto injector platform.


